Find a Gynaecologist
Find a GYNAECOLOGIST
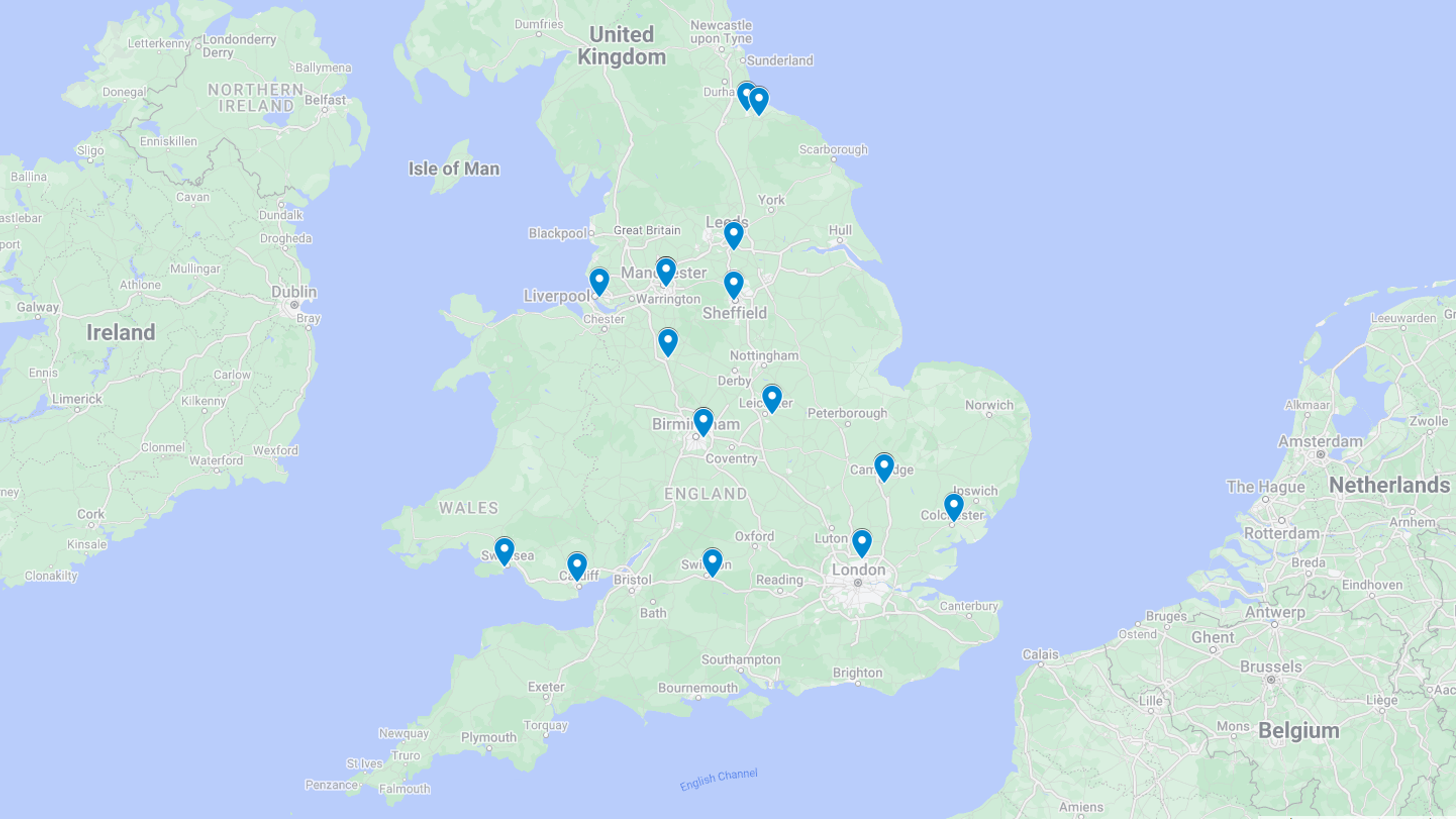
Find your nearest Hospital to make an appointment with a gynaecologist to discuss your options and suitability for the Sonata treatment.
All gynaecologists listed have agreed to appear on our website and have the proper equipment and training to offer the Sonata Treatment.
The Royal Victoria Infirmary
Queen Victoria Road
Newcastle Upon Tyne
NE14LP
Tel. 0191233 6161
Ms Michelle Russell
James Cook University Hospital
Marton Road
Middlesbrough
TS4 3BW
Tel. 01642 850850
Mrs Suzie Peatman
University Hospital of Hartlepool
Hardwick Road
Stockton-On-Tees
TS19 8PE
Tel. 01642 617617
Dr Dolonchampu Basu
Mr Roy
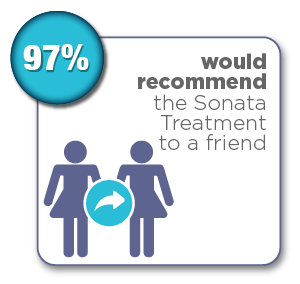
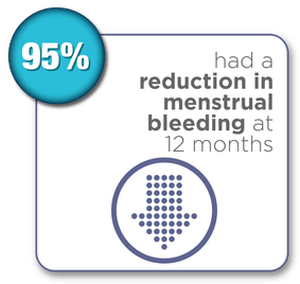
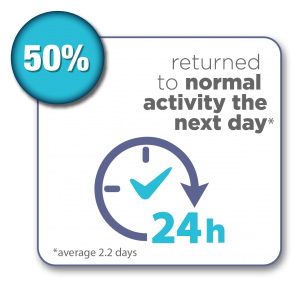
Women were surveyed 12 months after their Sonata Treatment and reported the following results:*
About the Sonata Treatment
The Sonata Treatment is an incision-free treatment for uterine fibroids and has been clinically proven to be safe and effective.*
The Sonata Treatment uses an intrauterine ultrasound handpiece to locate and target the individual fibroids. Radiofrequency energy is delivered to shrink the fibroid and reduce symptoms.
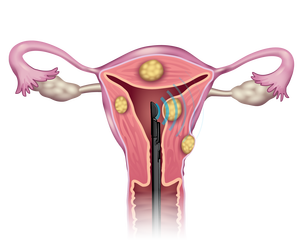
Why is the Sonata Treatment a breakthrough?
Sonata can treat a wide range of fibroid types, sizes, and locations — without even one incision.
The fibroids are treated from inside the uterus, so the Sonata Treatment requires no incisions to the abdomen, no tissue is surgically removed, and the uterus is preserved.
The SONATA Clinical Trial
Women were surveyed 12 months after their Sonata Treatment and reported the following results:*
- 97% would recommend Sonata treatment to a friend
- 95% reported a reduction in menstrual bleeding
- 50% returned to normal activity the next day, with an average of 2.2 days
Intended Use and Safety Information
The Sonata System is intended for diagnostic intrauterine imaging and transcervical treatment of symptomatic uterine fibroids, including those associated with heavy menstrual bleeding.
Contraindications
Current pregnancy; active pelvic infection; known or suspected gynecologic malignancy or premalignant disorders such as atypical endometrial hyperplasia; presence of one or more intratubal implants for sterilization; and presence of an intrauterine device (IUD), unless removed prior to the introduction of the Sonata Treatment Device. View Additional Safety Information.
* Reference for the published clinical trial results: Chudnoff S, Guido R, Roy K, Levine D, Mihalov L, Garza-Leal JG. Ultrasound-Guided Transcervical Ablation of Uterine Leiomyomas: The SONATA Trial. Obstet Gynecol. 2019 Jan; 133(1): 13-22
Safety Information | Impressum | Terms of Use | Contact Us
Privacy Notice | Cookie Notice | Do not Sell/Share my Personal Information
Limit the Use of My Sensitive Personal Information | Carbon Reduction Plan
Gynesonics, Inc. | 600 Chesapeake Drive | Redwood City, CA 94063 | (650) 216-3860
Copyright 2025 Gynesonics | WS 05195-002UK Rev C
![]()
![]()